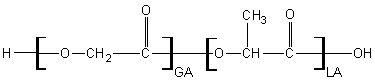
Poly(DL lactide-co-glycolide)
Description
PLGA is a random copolymer of lactide and glycolide. It is available in a wide range of both molecular weights and lactide to glycolide ratios. As the molecular weight increases degradation is slower due to many scissions being necessary to break down a long chain and as the lactide content increases the degradation is slower due to increased alkyl hydrophobication and potential to form crystalline domains. By convention, unless otherwise specified, PLGA is made utilizing DL lactide. Note that “Poly(Lactic-co-glycolic) acid” and “Poly(lactide-co-glycolide)” both refer to the same thing.
Poly(DL lactide-co-glycolide) polymers on PolySciTech.com
Full-text references
- Murphy, et. al., 2000, Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) sca!olds for tissue engineering (PDF)
- Jain, 2000, The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices (PDF)
- Laurencin, et. al. 2001, Poly(lactide-co-glycolide)/hydroxyapatite delivery of BMP-2-producing cells: a regional gene therapy approach to bone regeneration (PDF)
- Malloy, et. al. 1994, Induction of mucosal and systemic immune responses by immunization with ovalbumin entrapped in poly(lactide-co-glycolide) microparticles (PDF)
- Ravindran, et. al. 2010, Thymoquinone Poly(lactide-co-glycolide) Nanoparticles Exhibit Enhanced Anti-proliferative, Anti-inflammatory, and Chemosensitization Potential
- Wang, et. al, 2010, In vivo restoration of full-thickness cartilage defects by poly(lactide-co-glycolide) sponges filled with fibrin gel, bone marrow mesenchymal stem cells and DNA complexes (PDF)
- Shen, et. al. 2009, The bioactivity of rhBMP-2 immobilized poly(lactide-co-glycolide) scaffolds (PDF)