Site brought to you by PolySciTech
Poly(lactic acid)
a.k.a. poly(lactide)
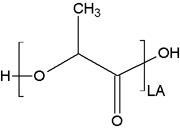
Description
Polylactide is a common polyester available by synthetic alteration of naturally occurring lactic acid.
Short chains can be made using polycondensation reactions (< 10k Da) however the MW of these is poorly controlled.
Conversion of the lactic acid to dimer form and ring-opening polymerization allows for generation of higher molecular weight polymers with greater control of the polymer molecular weight.
Due to the stereochemistry of the central carbon this polymer is present in both DL and L lactic forms.
The subtle difference in stereochemistry has a drastic impact on mechanical properties and degradation.
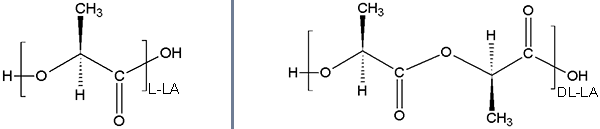
Poly(lactide) in L or DL racemic form.
Polylactic acid has a very useful property of naturally hydrolyzing back into lactic acid allowing it to be used medically for temporary implants and sutures.
Poly(lactic acid) polymers on PolySciTech.com
Full-text references
- Ahmed and Varshney, 2010, Polylactides—Chemistry, Properties and Green Packaging Technology: A Review (PDF)
- Puiggali, et. al. 2000, The frustrated structure of poly(l-lactide) (PDF)
- Bourges, et. al. 2003, Ocular Drug Delivery Targeting the Retina and Retinal Pigment Epithelium Using Polylactide Nanoparticles
- U. Edlund, A.-C. Albertsson, 2002, Degradable Polymer Microspheres for Controlled Drug Delivery (PDF)
- Gupta and Kumar, 2007, New emerging trends in synthetic biodegradable polymers – Polylactide: A critique (PDF)
FAQ
Q: Can you make other sizes, stereochemistries, and endcaps of polylactide?
A: Yes, this would be an extremely common polymer. The good news is we can typically fill these kinds of common requests in a 2-4 week delivery lead-time and, assuming you agree to allow the new product to become a catalog item, the price is the same as that of the other polylactides.